Pfizer covid vaccine safe for 5-yr olds – study
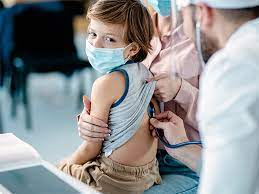
AFC – The Pfizer / BioNTech vaccine against Covid-19 is “safe” and “tolerated” by children aged 5 to 11 years, to whom the adapted dose generates a “robust” immune response, according to the results of a study announced Monday.
The vaccines administered to this group contain a lower dose, but generate a reaction “comparable” to that observed in patients between 16 and 25 years of age, they indicated in their statement.
They also stated that they will send this data to the authorities “as soon as possible.”
This is the first clinical data for this age group.
The drug regulatory agencies of the European Union and the United States had licensed Pfizer / BioNTech and Moderna vaccines, both based on messenger RNA technology, for 12 years.
Due to the spread of the delta variant, “since July, pediatric cases of Covid-19 had increased 240% in the United States, signaling the need for vaccination,” said Albert Bourla, CEO of Pfizer.
The doses of the drug in this group are 10 micrograms per injection, instead of the 30 given to older groups.
Its side effects are “generally comparable” to those seen in people ages 16 to 25, the labs noted.
They are partial results of a study carried out on 4,500 children between 6 months and 11 years old in the United States, Finland, Poland and Spain.
The two companies expect to publish in the fourth quarter the results of the range between 2 and 5 years and 6 months to two years, which received injections of 3 micrograms.





