State distributes hydroxychloroquine without research support
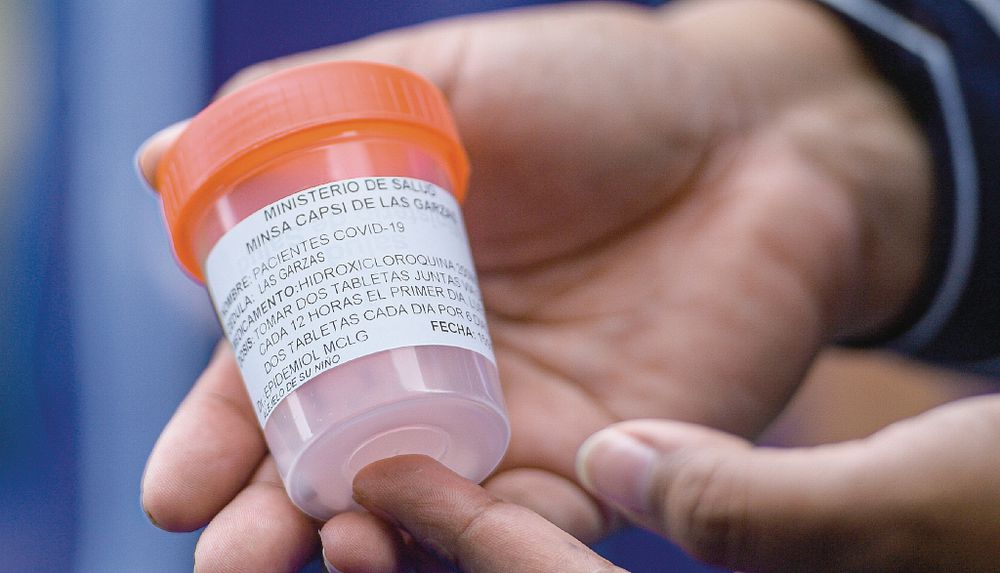
State distributes hydroxychloroquine without research support
During the 16 months that the pandemic has lasted, the National Research Bioethics Committee has not approved studies on the use of hydroxychloroquine for the treatment of Covid-19 but the State continues to distribute it reports La Prensa.
The studies that were presented were either withdrawn by the researchers or the procedures for their ethical approval were not completed.
In Panama, the debate on the use of hydroxychloroquine as an alternative for the prevention and treatment of Covid-19 disease persists, despite the “multiple pieces of evidence of its ineffectiveness” and recommendations from the World Health Organization, among other regulatory entities.
The Deputy Minister of Health, Ivette Berrío, said on Metro TV that the use of the drug is based on experience throughout the country, and on what is recommended by the epidemiology team and scientists, who according to her have had a favorable experience with the Medicines from the Protect yourself Panama kit.
On the website of the National Research Bioethics Committee ( CNBI) –an autonomous entity, there is no approved study on its use.
The president of the CNBI, Argentina Ying, Said that if there is no evidence, no drug should be used to treat the specific disease since all drugs used today must have undergone the rigor of research, which involves clinical trials in its phases.
If these studies were already done in other latitudes and it was shown that it does not meet the efficacy, doing the trial locally would not have social validity.
During the months of the pandemic, the Minsa and the Social Security Fund (CSS) have acquired at least 2,947,500 doses of hydroxychloroquine, for an approximate amount of $747,000, according to data from the Panama Compra portal.
The results of numerous clinical studies evaluating the efficacy of hydroxychloroquine against Covid-19, following an adequate clinical design, were conclusive in determining that it did not prevent or show greater benefit in people with positive PCR for Covid-19and and that since the end of last year the treatment guidelines for outpatients and hospitalized patients with Covid-19 have been known.
The WHO does not recommend using hydroxychloroquine to prevent Covid-19. This recommendation is based on six trials that included more than 6,000 participants without Covid-19 who were administered this drug, in which it was observed that administration had little or no effect in preventing this disease and hospitalization or death from it.
The WHO does not recommend using hydroxychloroquine to treat Covid-19. This recommendation is based on 30 trials that included more than 10,000 people who had contracted this disease since the drug did not reduce mortality or the need and duration of mechanical ventilation.
Taking hydroxychloroquine to prevent Covid-19 can increase the risk of diarrhea, nausea, abdominal pain, drowsiness, and headache
“If this drug is taken to treat Covid-19, it can increase the risk of suffering heart rhythm disturbances, blood, and lymphatic disorders, kidney injuries, and liver problems and falls,” said Dr Torres Atencio. The state, on the other hand, provided the medicine to all positive patients in the traceability process, and this was a great impediment, not counting all those citizens who self-medicated, just at the worst moment of the first wave.